Physical Chemistry - Chemical Thermodynamics
Complete Chemistry for Engg and Medical Entrance Exam Preparation. ( IIT JEE Main | Advanced | BITSAT | SAT | NEET etc.)

What you will learn
Explain the terms : system and surroundings
Discriminate between close, open and isolated systems
Explain internal energy, work and heat
State first law of thermodynamics and express it mathematically
Calculate energy changes as work and heat contributions in chemical systems
Explain state functions: U, H
Correlate ∆U and ∆H
Measure experimentally ∆U and ∆H
Define standard states for ∆H
Calculate enthalpy changes for various types of reactions
State and apply Hess’s law of constant heat summation
Differentiate between extensive and intensive properties
Define spontaneous and nonspontaneous processes
Explain entropy as a thermodynamic state function and apply it for spontaneity
Explain Gibbs energy change (∆G)
Establish relationship between ∆G and spontaneity, ∆G and equilibrium constant
Why take this course?
SUMMARY
Thermodynamics deals with energy changes in chemical or physical processes and enables us to study these changes quantitatively and to make useful predictions. For these purposes, we divide the universe into the system and the surroundings. Chemical or physical processes lead to evolution or absorption of heat (q), part of which may be converted into work (w). These quantities are related through the first law of thermodynamics via ∆U = q + w. ∆U, change in internal energy, depends on initial and final states only and is a state function, whereas q and w depend on the path and are not the state functions. We follow sign conventions of q and w by giving the positive sign to these quantities when these are added to the system. We can measure the transfer of heat from one system to another which causes the change in temperature. The magnitude of rise in temperature depends on the heat capacity (C) of a substance. Therefore, heat absorbed or evolved is q = C∆T. Work can be measured by w = –pex ∆V, in case of expansion of gases. Under reversible process, we can put pex = p for infinitesimal changes in the volume making wrev = – p dV. In this condition, we can use gas equation, pV = nRT.
At constant volume, w = 0, then ∆U = qV , heat transfer at constant volume. But in study of chemical reactions, we usually have constant pressure. We define another state function enthalpy. Enthalpy change, ∆H = ∆U + ∆ngRT, can be found directly from the heat changes at constant pressure, ∆H = qp .
There are varieties of enthalpy changes. Changes of phase such as melting, vaporization and sublimation usually occur at constant temperature and can be characterized by enthalpy changes which are always positive. Enthalpy of formation, combustion and other enthalpy changes can be calculated using Hess’s law.
First law of thermodynamics does not guide us about the direction of chemical reactions i.e., what is the driving force of a chemical reaction. For isolated systems, ∆U = 0. We define another state function, S, entropy for this purpose. Entropy is a measure of disorder or randomness. For a spontaneous change, total entropy change is positive. Therefore, for an isolated system, ∆U = 0, ∆S > 0, so entropy change distinguishes a spontaneous change, while energy change does not. Entropy changes can be measured by the equation ∆S = qrev/T for a reversible process. qrev/T is independent of path.
1 Choose the correct answer. A thermodynamic state function is a quantity (i) used to determine heat changes (ii) whose value is independent of path (iii) used to determine pressure volume work (iv) whose value depends on temperature only.
2 For the process to occur under adiabatic conditions, the correct condition is: (i) ∆T = 0 (ii) ∆p = 0 (iii) q = 0 (iv) w = 0
3 The enthalpies of all elements in their standard states are: (i) unity (ii) zero (iii) < 0 (iv) different for each element
4 The enthalpy of combustion of methane, graphite and dihydrogen at 298 K are, –890.3 kJ mol–1 –393.5 kJ mol–1, and –285.8 kJ mol–1 respectively. Enthalpy of formation of CH4 (g) will be
(i) –74.8 kJ mol–1 (ii) –52.27 kJ mol–1 (iii) +74.8 kJ mol–1 (iv) +52.26 kJ mol–1 .
5 A reaction, A + B → C + D + q is found to have a positive entropy change. The reaction will be (i) possible at high temperature (ii) possible only at low temperature (iii) not possible at any temperature (v) possible at any temperature
6 In a process, 701 J of heat is absorbed by a system and 394 J of work is done by the system. What is the change in internal energy for the process?
7 Calculate the number of kJ of heat necessary to raise the temperature of 60.0 g of aluminium from 35°C to 55°C. Molar heat capacity of Al is 24 J mol–1 K–1 .
8 Enthalpy of combustion of carbon to CO2 is –393.5 kJ mol–1. Calculate the heat released upon formation of 35.2 g of CO2 from carbon and dioxygen gas.
9 Enthalpies of formation of CO(g), CO2 (g), N2O(g) and N2O4 (g) are –110, – 393, 81 and 9.7 kJ mol–1 respectively. Find the value of ∆rH for the reaction: N2O4 (g) + 3CO(g) → N2O(g) + 3CO2 (g)
10 For an isolated system, ∆U = 0, what will be ∆S ?
11 For the reaction at 298 K, 2A + B → C ∆H = 400 kJ mol–1 and ∆S = 0.2 kJ K–1 mol–1 At what temperature will the reaction become spontaneous considering ∆H and ∆S to be constant over the temperature range.
12 For the reaction, 2 Cl(g) → Cl2 (g), what are the signs of ∆H and ∆S ?
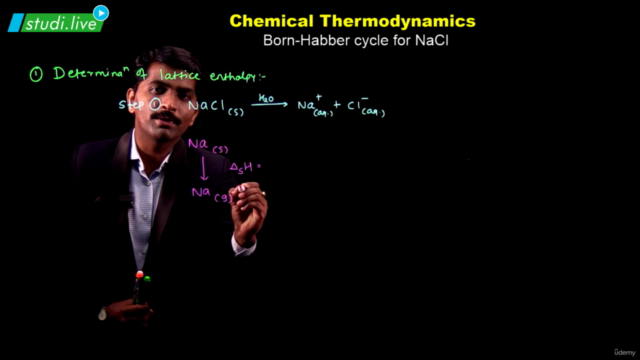
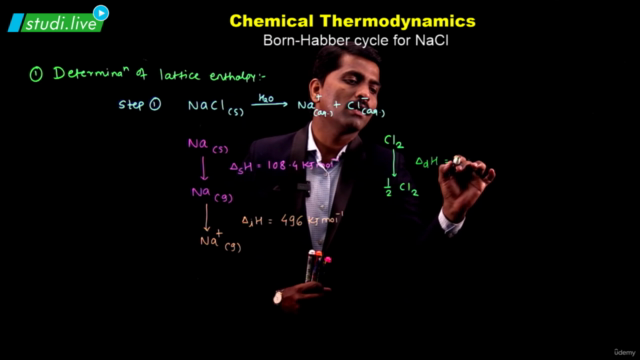
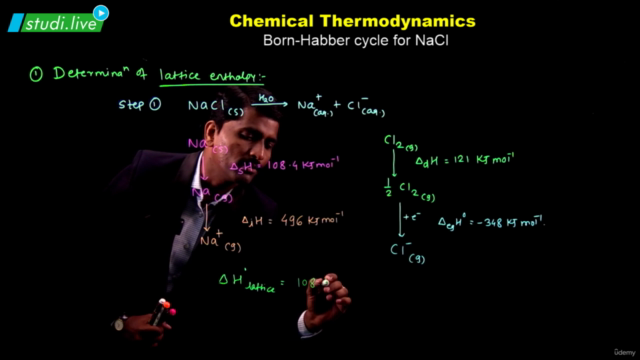
Screenshots

Charts
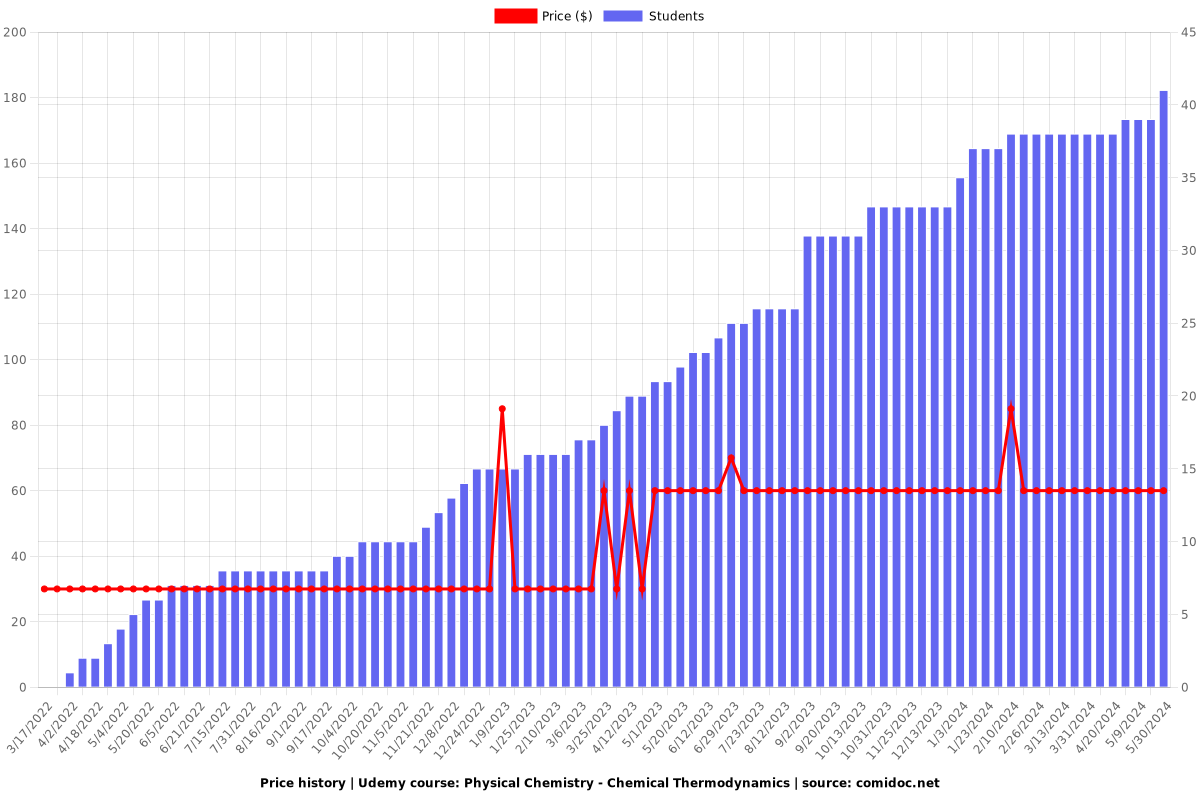
Price
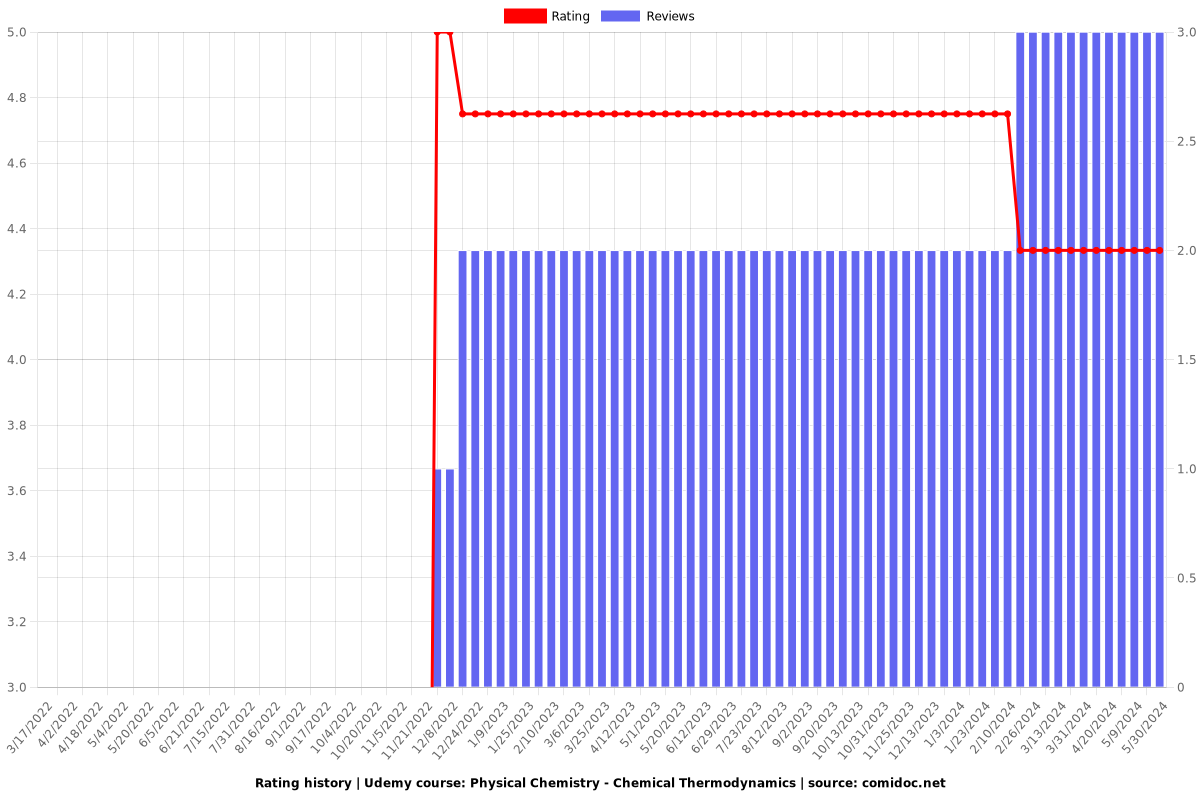
Rating
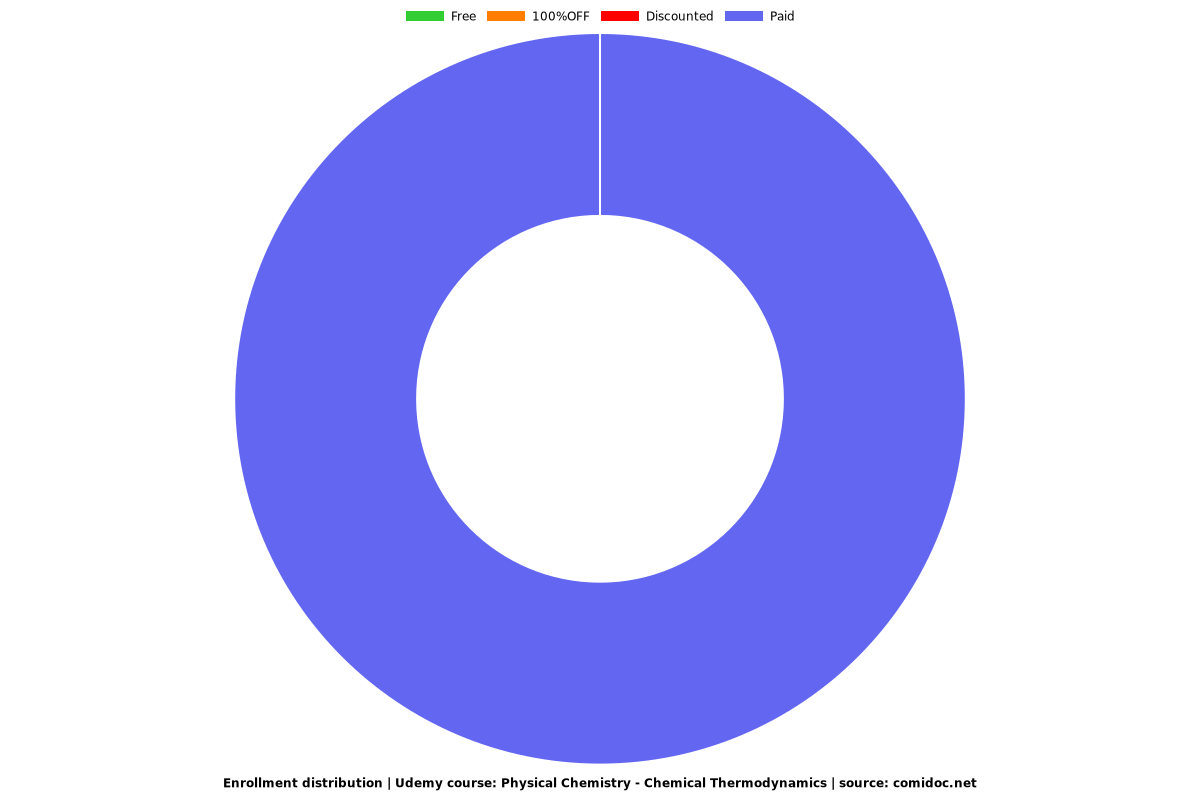
Enrollment distribution