Title
Modern Medical Device Development
Phases of medical device development, Medical device safety and compliance, How to train your staff on medical device.
What you will learn
Understanding the phases of medical device development
Medical device safety and compliance
How to train your staff on new medical device
Single - use medical device reprocessing
Development of medical device policies
Medical device regulation act
Home medical equipment
How to sterilize medical instruments
Why take this course?
Course Headline: 🏥 Master the Art of Modern Medical Device Development with Expert Eric Yeboah!
Course Title: Modern Medical Device Development
Introduction: Welcome to "Modern Medical Device Development," a comprehensive course designed to guide you through the intricate process of creating safe, effective, and compliant medical devices. In this dynamic field, where technology evolves at a staggering pace, understanding the phases of development, ensuring safety and compliance, and effectively training your staff are critical components for success. Join us as we delve into the critical aspects of medical device manufacturing with industry expert Eric Yeboah.
Course Description: 🔹 The Importance of Safety and Compliance: In the realm of medical devices, safety is paramount. With recent regulations tightening to ensure patient and user risk is minimized, it's crucial for manufacturers to integrate quality and eliminate risks from their devices. This course will explore the complexities of advanced testing and certification processes necessary to verify a device's safety, performance, and regulatory compliance.
🔹 Phases of Medical Device Development: From concept to market, we will take you through each phase of medical device development. Learn about design controls, risk management, usability engineering, and the critical steps in bringing a product from ideation to production.
Key Topics Covered:
- Understanding the regulatory landscape for medical devices globally
- Strategies for developing safe and effective medical devices
- The role of standards in ensuring patient safety and data security
- Best practices for compliance with ISO, FDA, and CE marking requirements
- Techniques for effective staff training on medical device technologies
🔹 Navigating Regulatory Compliance: The world of medical device regulation can be overwhelming. This course will demystify the process, helping you understand the latest trends, standards, and policies that ensure your products not only comply but also contribute positively to global health objectives.
Why Take This Course?
- Expertise: Learn from the insights of Eric Yeboah, a seasoned professional with extensive experience in medical device development.
- Comprehensive Learning: Gain a deep understanding of the entire lifecycle of a medical device, from concept to patient care.
- Practical Skills: Acquire the skills necessary to ensure your medical devices are safe, effective, and compliant with all necessary regulations.
- Global Impact: Understand how your work contributes to healthcare innovation and addresses critical health needs worldwide.
Who Should Enroll? This course is ideal for:
- Medical Device Engineers
- Regulatory Affairs Professionals
- Quality Assurance Specialists
- Clinicians and Healthcare Providers
- Entrepreneurs and Startups in the MedTech space
- Anyone interested in the healthcare industry and medical device development
Enroll Today! Embark on your journey to becoming a medical device development expert. Enhance your understanding of safety, compliance, and staff training with our engaging course material. Secure your spot now and transform the future of healthcare with safe, innovative medical devices!
Screenshots




Reviews
Charts
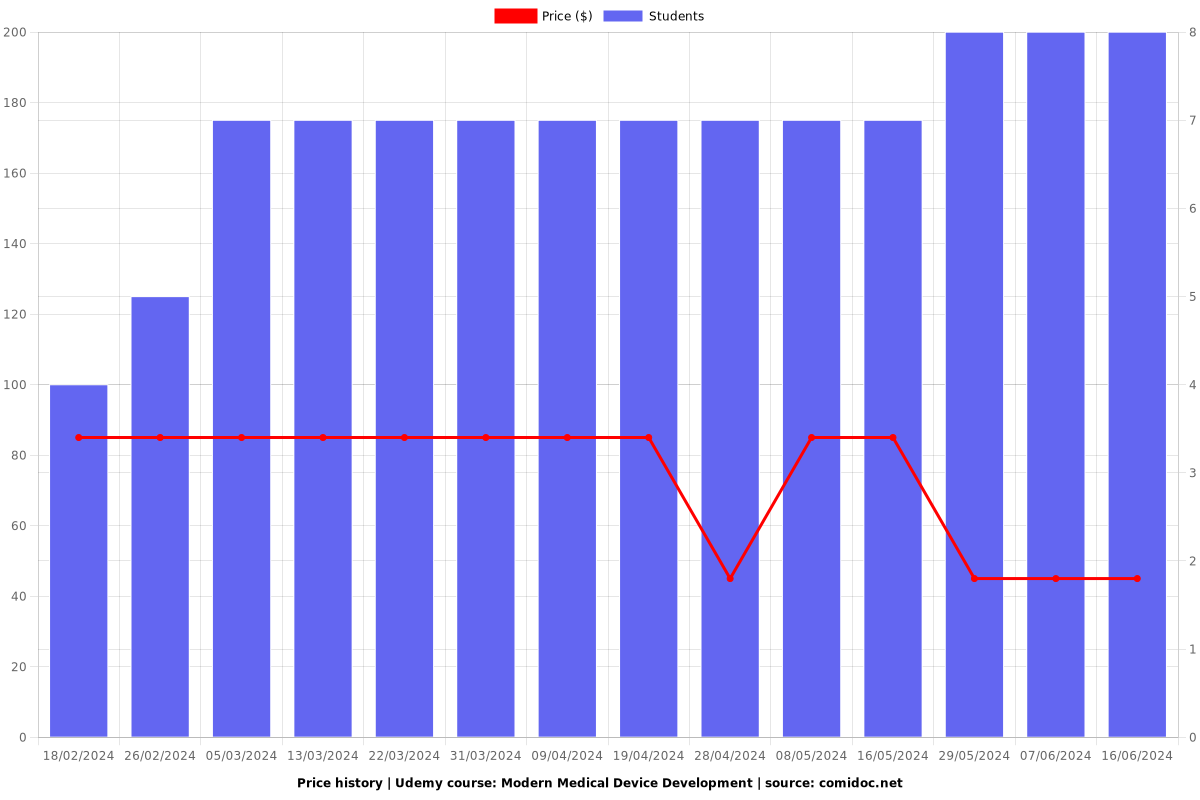
Price
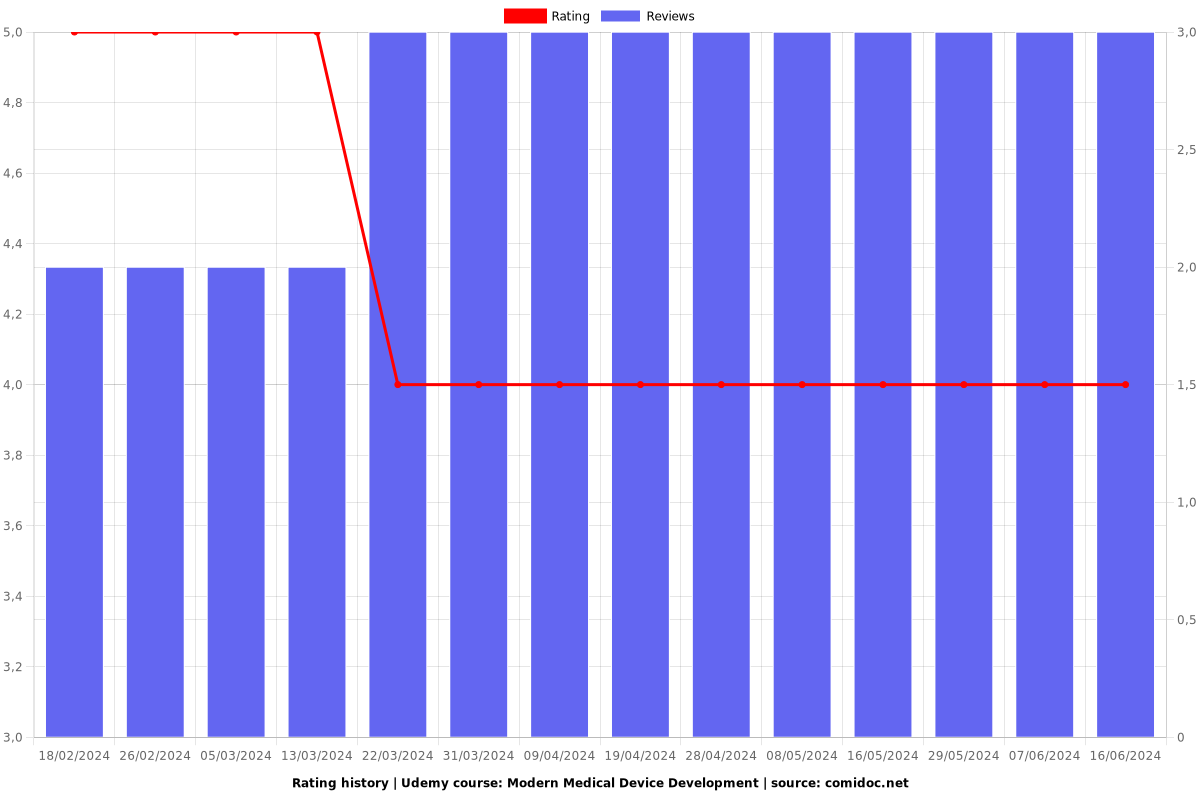
Rating
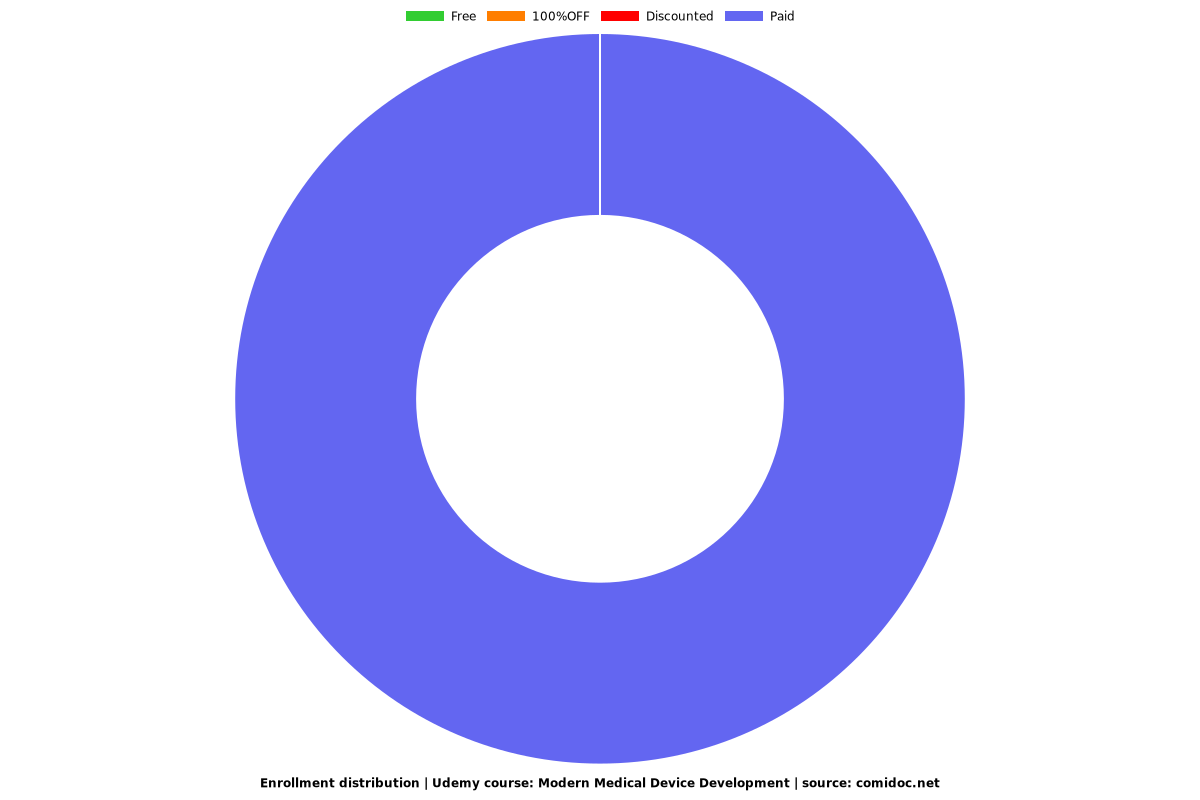
Enrollment distribution