Master medical devices registration in Canada
Manage all regulatory activities of medical devices in the market of Canada

What you will learn
Master Medical devices registration in Canada
Manage all regulatory activities of medical devices in Canada
Place your medical devices in the market of Canada
Master the medical devices market of Canada
Why take this course?
Master medical devices registration in the most important and biggest market ever in the world, the market of Canada, this market could be missed by any pharmaceutical or healthcare company, all the pharma industry professionals understand very well the weight of this market, how huge it is, and what is the expected revenue out of it.
This course will equip you with two powerful tools to invade the pharma industry, as a regulatory affairs professional you are responsible for bringing up to more than half of the revenue for such a company on one hand while on the other hand, you are dealing with the second powerful tool of the medical devices, as medical devices registration time frame is shorter than the pharmaceuticals, it is almost half the time frame of the pharmaceuticals.
To register a medical device you will need only 6 months, 3 months for variation, and 2 to 1 month only for renewal.
So from a business point of view, you can place your product in the market quite faster and start earning your revenue quite fast also, moreover, the medical devices world is highly dynamic and innovative, compared to the pharmaceuticals, with lots of innovations and different versions of the same medical device could be played within the potential market of Canada.
Dear pharma industry future professionals, let us start our interesting journey toward medical device registration in the states, we will travel together to the states, meet the health authority there "HC", and explore its requirements for registration, renewal, and variations, let us get our "HC" approval and place our products there.
In this course, we will cover all topics related to medical devices in Canada, starting from the requirements, interim regulations, classification, verification, special conditions, and weavings
And finally, congratulations to you for mastering medical device registration in the Canadian market, and see you soon in the pharma industry my dear colleagues.
Screenshots




Reviews
Charts
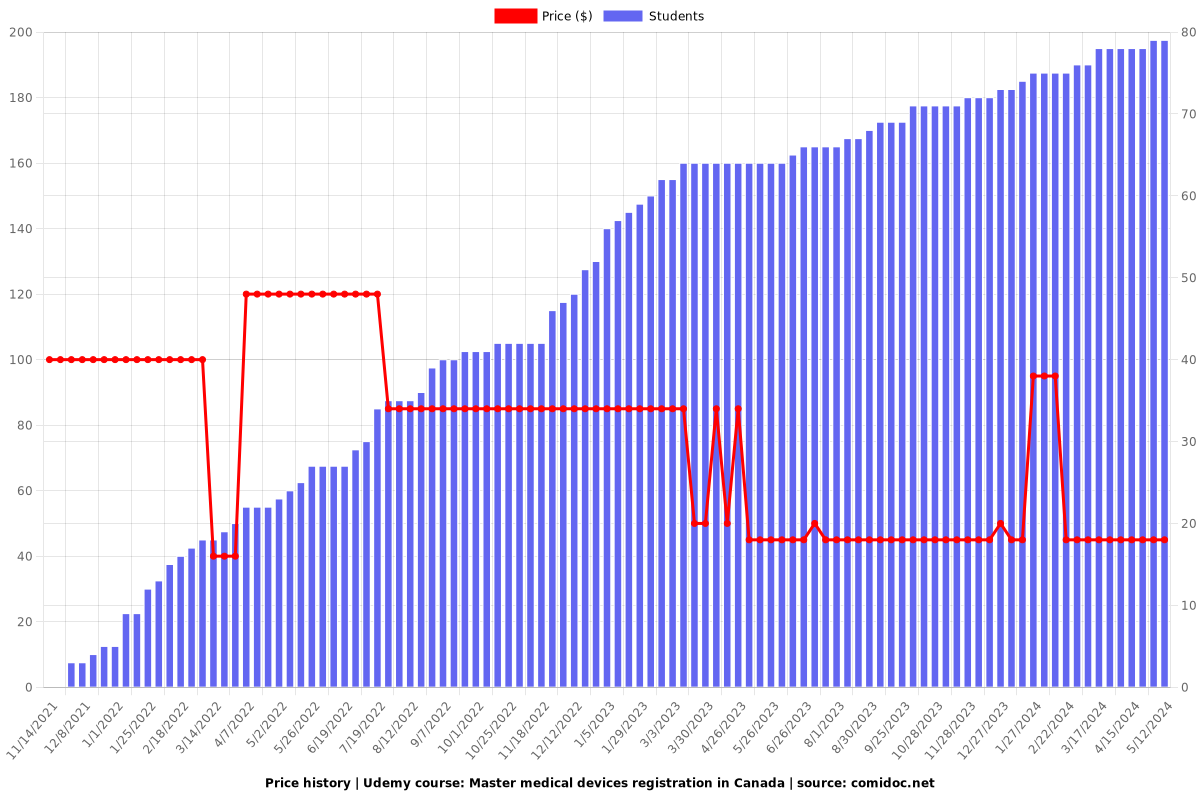
Price
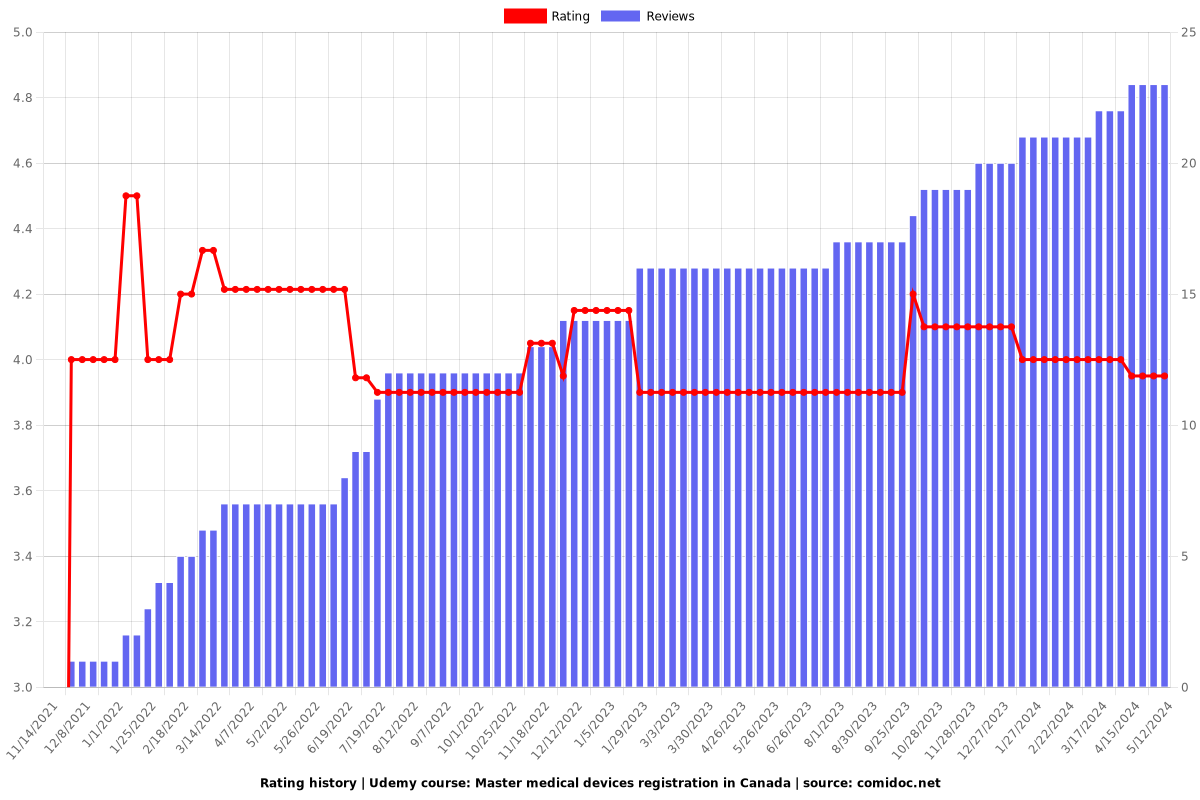
Rating
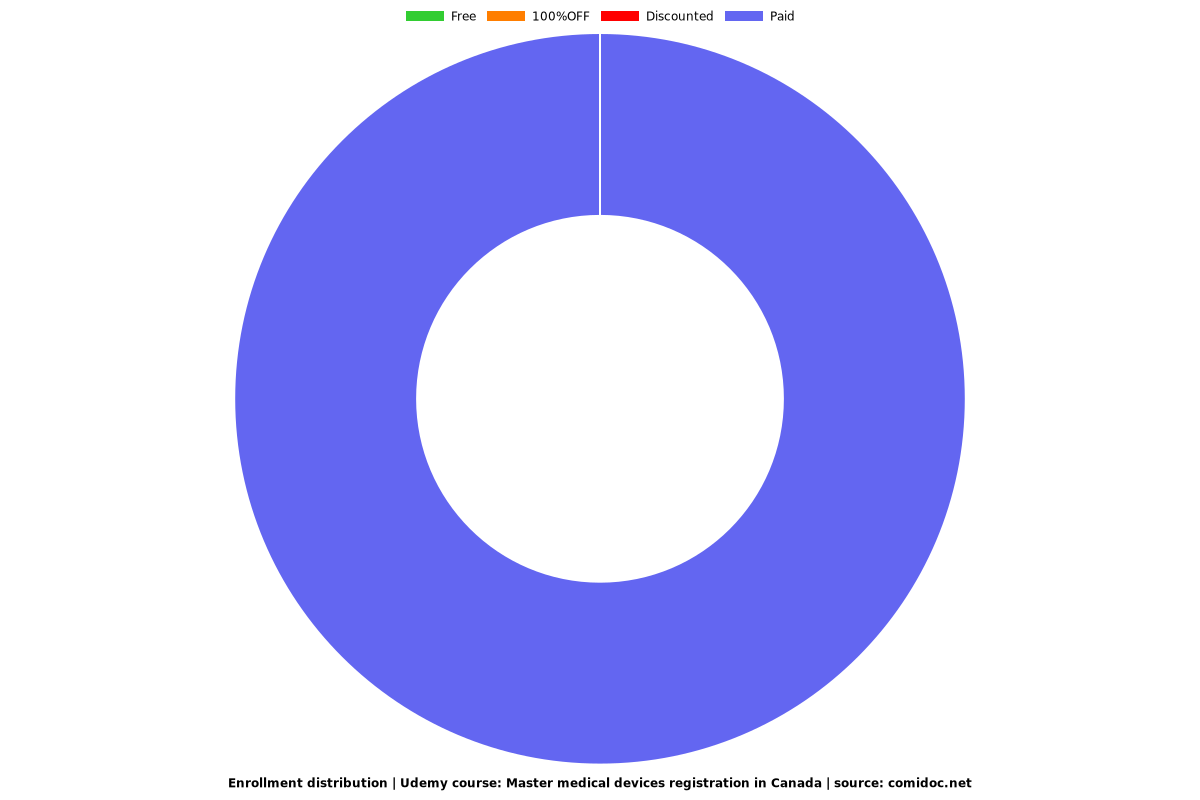
Enrollment distribution