Title
ISO 14971:2019 Risk Management for Medical Devices
Application Risk Management for medical devices

What you will learn
How ISO 14971:2019 Risk management
Risk management Policy
FMEA and it's type
Hazard identification and types
Risk Benefit Analysis
Fault tree analysis
Risk Management Plan
Probability Estimation of Hazards
How to Use 5 × 5 Matrix
Residual Risks
Risk Acceptability Creteria
Risk Mitigation
Case studies of FMEA
Case Study of Risk Management
Documents of Risk Management File
Risk Control Measures
Why take this course?
🚑 ISO 14971:2019 Risk Management for Medical Devices 🚀
Course Headline: Application of Risk Management for Medical Devices
Course Description:
Risk Management is a cornerstone in ensuring compliance with the Quality Management system requirements as stipulated by the ISO 13485 Standard. This critical process is essential for medical device and In-vitro Diagnostic (IVD) device manufacturers, as it safeguards patient safety, optimizes product design and development, and ultimately ensures that devices perform safely and effectively throughout their life cycle.
Why is this important? Analyzing the root causes of product failures often points to inadequacies in risk management practices within organizations. Recognizing the importance of this discipline, this training course aims to enhance your organization's understanding and proficiency in implementing effective Risk Management strategies as per ISO 14971:2019.
Course Objectives:
This comprehensive course is tailored to provide you with a robust understanding of the Risk management Standard and its profound impact on the medical device industry. You will gain insights into how risk management influences decision-making processes in the manufacturing of medical devices, thereby enhancing your organization's ability to manage risks effectively.
What you will learn:
🔹 Identify the key requirements of ISO 14971:2019 and ensure your organization meets these standards.
🔹 Interpret and communicate the expectations of ISO 20417:2021 effectively to your stakeholders and team members.
🔹 Understand the interrelation between ISO 14971:2019, ISO 13485, and the MDR (Medical Devices Regulation) 2017/745 for a cohesive approach to quality management and risk control.
🔹 Learn how to apply the fundamental risk management activities within your organization, ensuring a systematic and proactive approach to managing risks associated with medical devices.
What You Will Learn:
Upon completion of this training, you will be equipped to:
-
📚 Define key risk management terminology used in the industry, enhancing clear communication within your organization.
-
⏰ Explain how risk management fits into the product lifecycle, ensuring a comprehensive approach to safety and efficacy throughout the device's life.
-
📈 Outline the stages of the risk management process, from hazard identification to risk evaluation, analysis, and mitigation strategies.
-
📑 Define the key deliverables expected from a robust Risk Management process, including Risk Management Files (RMF), and understand their importance in compliance and safety assurance.
-
🏭 Apply risk management principles within your organization, fostering a culture of quality, safety, and reliability in medical device development and manufacturing.
Join our course and step into the world of effective Risk Management for Medical Devices, where you will gain the knowledge and tools to ensure your products are not just compliant but also safe and reliable for end-users. Let's embark on this journey to elevate the standards of medical device manufacturing together! 🎓🩺✨
Screenshots




Our review
Course Review: [Course Name]
Overall Rating: 3.66 out of 5
Pros:
- The course content provides detailed information on risk management and application in real-time scenarios, which is valuable for learners.
- Some users found the content to be good despite the audio issues.
- The pace of the course allows for a comprehension of the subject matter, assuming the audio quality was not an issue.
Cons:
-
Audio Quality: Multiple reviews indicate significant echo in all audio tracks, which combined with the speaker's accent, makes understanding the content difficult. This is a critical issue that detracts from the learning experience.
- Echo present in all audio tracks
- Accent may complicate comprehension
-
Presentation and Teaching Style: The presenter reads directly from the slides, indicating a lack of engaging teaching methods. There is an expectation for a native speaker to improve clarity.
-
Content Depth and Substance: Several users felt that the course was too superficial, suggesting that more in-depth notes or supplementary materials would be beneficial for learners to study further post-course.
-
Grammar and Language Accuracy: Grammatical errors are present on the slides, and there are issues with the accuracy of the translation provided.
-
Quizzes: Quizzes are criticized for having predictive answers that almost always result in a "yes," and sometimes the questions do not align with the course content. Additionally, quiz questions themselves are often too short to effectively assess understanding.
-
Quality of Materials: The example spreadsheet included in the slides is reportedly so blurry that it's difficult to read, which affects the practical application of the course lessons.
-
Transcript Verification: There is a need for verification of transcripts against the actual audio to ensure accuracy as several discrepancies were noted by learners.
Course Structure and Presentation:
- The course is structured in a way that presents detailed information, but this is marred by significant technical issues such as poor audio quality and read-only presentations.
- Despite these flaws, some users still found the content to be good, indicating there is value in the subject matter being taught.
- It is recommended that the course organizers prioritize improving audio recording quality and presentation techniques to enhance the learning experience.
- Additionally, it would be beneficial to provide clearer slide materials and more comprehensive study aids to complement the course content.
In conclusion, while there is potential for [Course Name] to be an effective educational tool, it is currently hindered by technical issues and superficial treatment of complex topics. Addressing these shortcomings could significantly increase the course's value and effectiveness.
Note to Course Administrators: It is crucial to address the audio quality and presentation style immediately to improve the learner experience. Additionally, ensuring that all materials are clear and accurate, and that quizzes effectively measure understanding, will enhance the overall quality of the course. Providing comprehensive notes or supplementary materials for further study would also be a valuable addition.
Charts
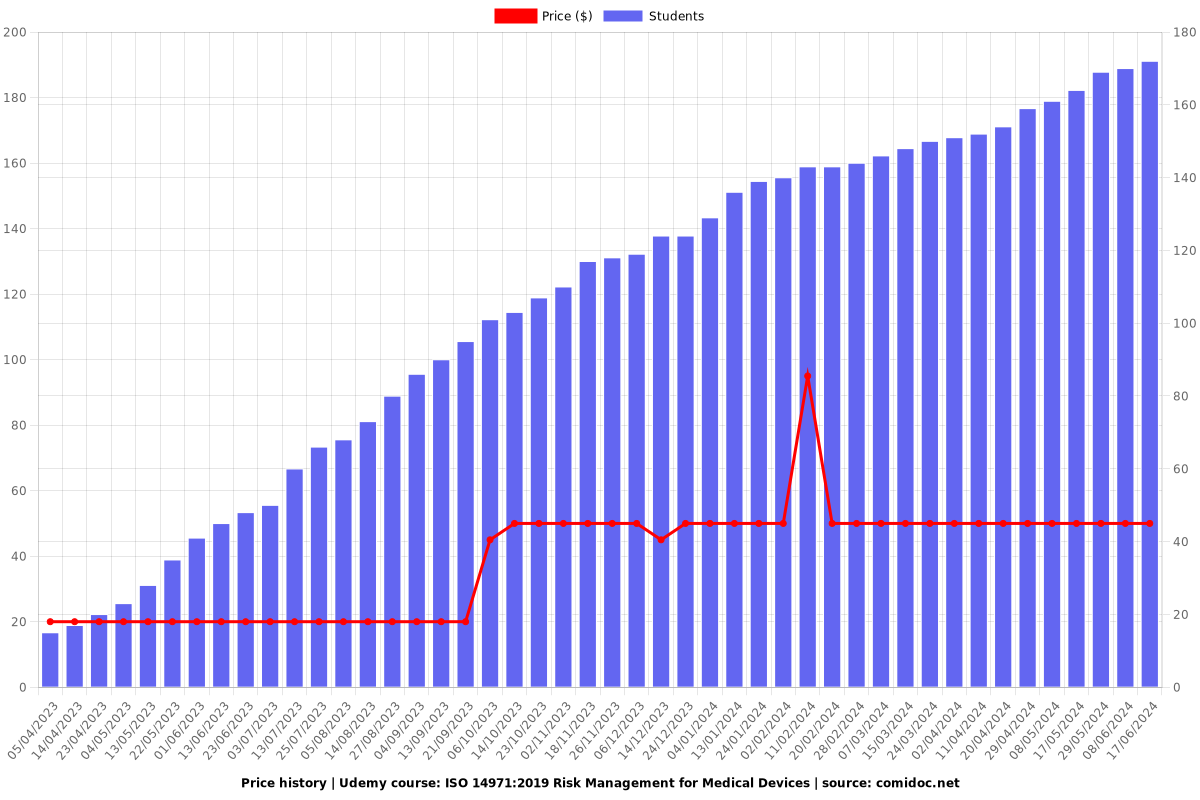
Price
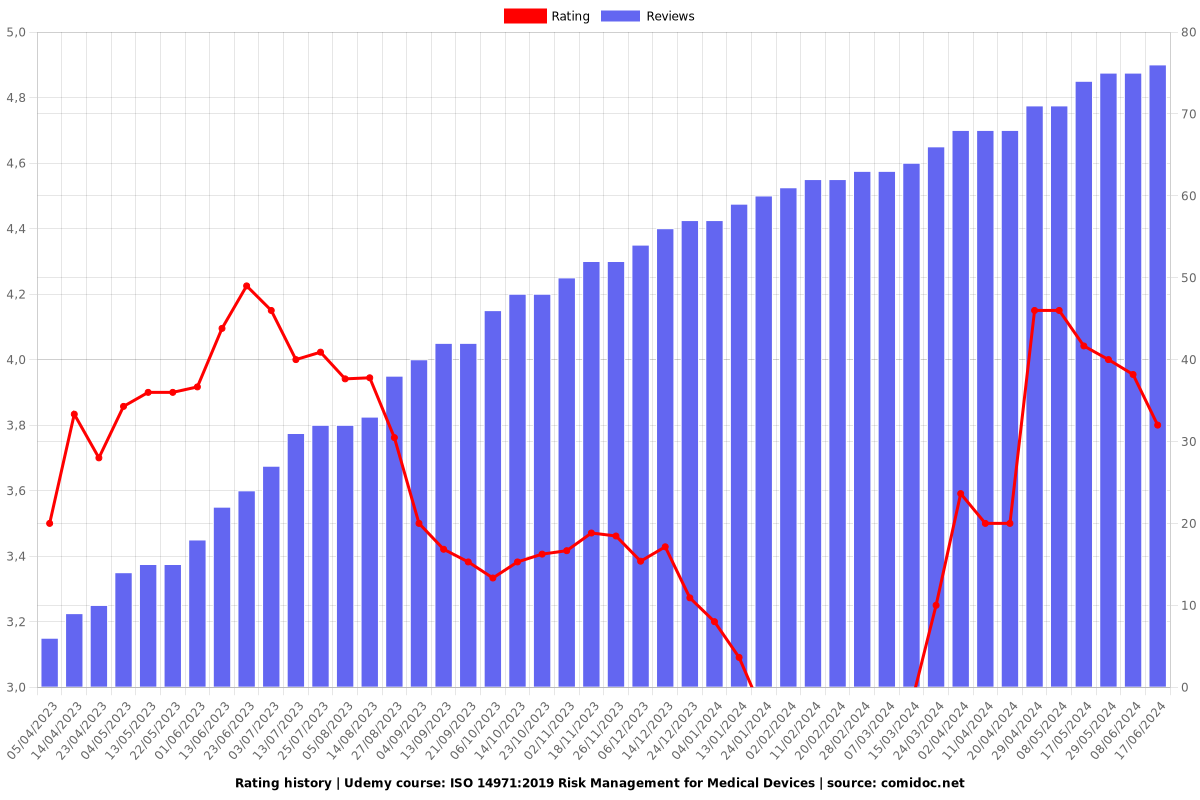
Rating
Enrollment distribution